Need Help?
Do not hesitate to contact us. for any order of our Miracle Tea.
visionsmapa@gmail.com
(480) 386-7004
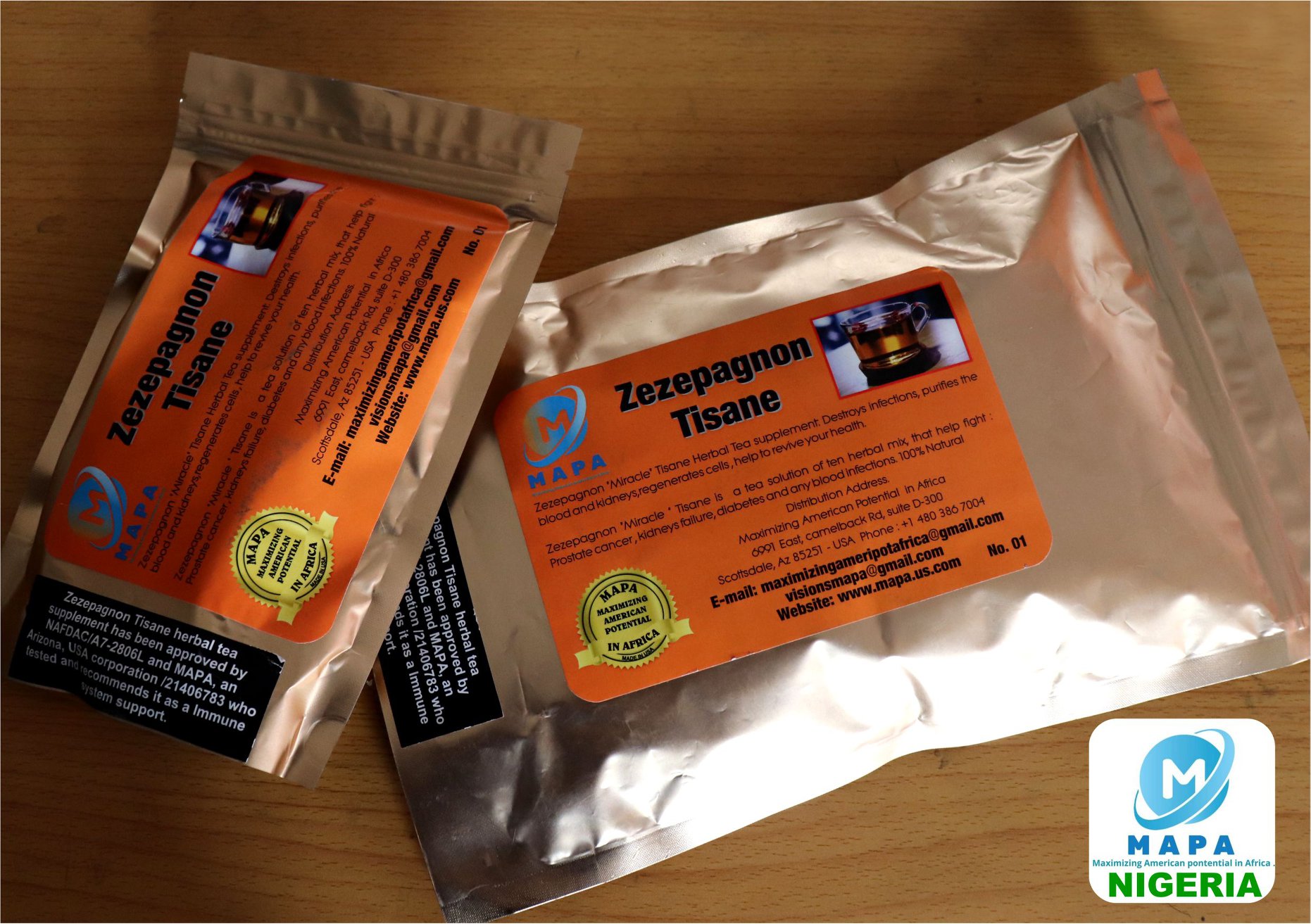
Zezepagnon “Miracle” Tisane Herbal Tea Supplement
Zezepagnon “Miracle” Tisane is a tea comprised of ten all-natural herbs that promote your health by helping to fight prostate Cancer, and diabetes as well as improve failing kidneys and urinary tract infections in Men and Women.
All diseases are caused by what we eat. Specifically, Prostate Cancer, Kidney Failure, Diabetes and Urinary Tract Infections are caused by three things: Unhealthy Foods, Over indulgence in Alcohol or Sugar, and by Infections. Once in your blood system is polluted with these harmful substances it will begin to carry microbes that will poison the body and weaken organs such as the Pancreas. To cleanse your body of these toxins it is necessary to practice healthy eating habits and overall change your behavior, but also it is necessary to aid your system’s ability to purify itself. Today,Diabetes, Prostate Cancer and
Kidney Failure are killing people at alarming numbers. Tisane Herbal Tea, also known as Zezepagnon “Miracle”, is an all natural supplement found to help improve
The composition of the tea
Zezepagnon is an herbal tea manufactured and distributed in the USA as a nutritional supplement with an immuno-stimulating power. It is a summary of a dozen medicinal and nutritional plants from Africa, USA, Mexico and Brazil.


Pharmacological properties
The multiplicity and diversity of plants that make up Zezepagnon is at the origin of a synergy of action of several natural molecules inducing the following biological effects:
- Stimulating the immune system allowing the body to defend itself against infections and cancers,
- Healing internal and external to heal external wounds, haemorrhages and ulcers,
- Anti-infectious against all forms of infections and germs resistant to current antibiotics,
- Antiviral against all forms of viruses
- Anti-inflammatory to cure all kinds of inflammations,
- Regenerating and energizing nerves and muscles to restore motor functions disrupted by an accident or aging
- Regenerating and energizing nerves and muscles to restore motor functions disrupted by an accident or aging Glucose regulator
- Regulator of arterial blood pressure
- Diuretic to quickly evacuate excess water and toxins from the body
- Aphrodisiac to correct erectile dysfunctions
The Indications of the tea
– Cancers: throat, colon, uterus, pancreas, liver, prostate, breast, blood, brain, stomach, muscle, bone, bladder, kidney, testicles
– Viral diseases: AIDS, hepatitis, arboviruses
– Infectious diseases: Malaria, eruptive diseases, tuberculosis, leishmaniasis,
– Metabolic diseases: Diabetes, gout, endocrine diseases, osteoporosis, dyslipidemia
– Osteo articular diseases: rheumatoid arthritis, arthritis, osteoarthritis …
– Skin diseases: scars, herpes, onychomycosis, eczema, psoriasis, shingles ….
– Neurological diseases: Alzheimer, Parkinson, Epilepsy
– Diseases of the kidneys and the urinary system: Renal lithiases, cystitis, acute and chronic renal failure, benign hypertrophy of the prostrate, enuresis
– Respiratory diseases and the ENT sphere: cystic fibrosis, asthma, tuberculosis, chronic obstructive pulmonary disease, sinusitis ….
– Gynecological diseases: ovarian cysts, uterine fibroids, vaginitis, dysmenorrhea, condyloma ….
– diseases of the eye: Glaucoma, cataracts, myopia, presbyopia, ….
– Mental illnesses: Schizophrenia, depression, insomnia ……
– Diseases of the circulatory system: stroke, high blood pressure, pulmonary embolism, phlebitis, varicose veins …..
– Autoimmune diseases: Lupus, multiple sclerosis ….
– Diseases of the digestive system: gallstones, gastroduodenal ulcer, functional disorders of the intestine
– Genetic diseases: sickle cell disease
Marketing rights
Scientific Research Projects on Zezepagnon
Pre-clinical research concerning experiments to be performed at the Laboratory. Thus, the pharmacological properties suspected of Zezepagnon will be evaluated by methods already validated in the Laboratory: it will be mainly antioxidant, anti-inflammatory, analgesic, antipyretic, anticancer, anti-infectious, antiviral, diuretic, anti-HTA, regulatory glycemia, stabilizing biochemical parameters, immunostimulant, healing
– Galenic formulation of Zezepagnon
In the medium term, it will be a question of putting on the market a modern galenic form of Zezepagnon easily transportable, and having the same pharmacological properties as the form herbal tea.
To do so, we will make a 15-minute decoction of Zezepagnon respecting the initial proportions of water / vegetable matter. After filtration, the resulting liquid will be lyophilized to obtain a powder. Zezepagnon lyophilisate will be used to make effervescent tablets or capsules. The dosage of these tablets or capsules will be 2Cp / day or 2Gel / day, 1 to noon and 1 in the evening, to dissolve in 1/2 glass of water (250mL)
– Clinical research
Clinical research concerns work in hospitals near patients. It will initially be necessary to prove the ethno-medical evidence of Zezepagnon from a cohort of patients according to the targeted pathology who will receive the product according to the recommended posology and will be followed by a doctor specialized in this pathology. The symptomatic evolution as well as that of the biochemical parameters of the patient will be followed by the specialist doctor for a duration of 1-3 months according to the nature and gravity of the pathology.
In a second step, once the dosage form of Zezepagnon will be available, it will be necessary to carry out a clinical study properly speaking, respecting the different phases recommended by WHO.
Implementation Strategy
Once the finances are in place, a pilot laboratory will be set up to evaluate the different pharmacological properties of Zezepagnon.
Master 2 and PhD students will be recruited to carry out pre-clinical research and their results will be supported by scientific publications and publications in PubMed-indexed journals with a high impact factor. This will introduce Zezepagnon into the academic world of high level.
The resultant database of this research will move Zezepagnon from a food supplement to a market authorization (MA).
In the long term, it will be necessary to set up a pilot medical center that will include consultations and medical tests as well as hospitalizations so that the patient can be followed completely until recovery.
These pilot projects will be carried out in an African country chosen by MAPA’s international manager. If the results of the pilot projects are conclusive, other laboratories and medical centers can be established in other countries of the world as needed.


